Sulfo-EMCS crosslinker
| Cat. # | Quantity | Price | Lead time | Buy this product |
|---|---|---|---|---|
| PCL036_0050 | 50 mg | Enquire | in stock | |
| PCL036_0100 | 100 mg | Enquire | in stock |
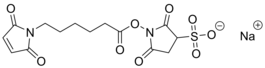
- IUPAC Name: N-ε-maleimidocaproyl-oxysulfosuccinimide ester
- Synonyms: Sulfo-EMCS, (N-(ε-maleimidocaproyloxy) sulfosuccinimide ester)
- CAS: 103848-61-9 (of acid)
- Smiles: O=C(CC1S(=O)([O-])=O)N(OC(CCCCCN2C(C=CC2=O)=O)=O)C1=O.[Na+]
- Chemical formula: C14H16N2NaO9S
- Molecular weight: 410.33
- Purity: 95%+
EMCS and its water-soluble analog Sulfo-EMCS are heterobifunctional cross-linkers that contain N-hydroxysuccinimide (NHS) ester and maleimide groups that allow covalent conjugation of amine- and sulfhydryl-containing molecules. NHS esters react with primary amines at pH 7-9 to form amide bonds, while maleimides react with sulfhydryl groups at pH 6.5-7.5 to form stable thioether bonds. In aqueous solutions, hydrolytic degradation of the NHS ester is a competing reaction whose rate increases with pH. The maleimide group is more stable than the NHS-ester group but will slowly hydrolyze and also lose its reaction specificity for sulfhydryls at pH values > 7.5. For these reasons, conjugation experiments involving these cross-linkers are usually performed at pH 7.2-7.5, with the NHS-ester (amine-targeted) reaction being accomplished before or simultaneous with the maleimide (sulfhydryl-targeted) reaction.
Features and Benefits
- Reactive groups: sulfo-NHS ester and maleimide
- Reactive towards: amino and sulfhydryl groups
- Sulfo-NHS ester end couples with primary amines at pH 7-9 to form stable amide bonds
- Maleimide reacts with -SH groups at pH 6.5-7.5, forming stable thioether linkages
- Non-cleavable
- Water-soluble (compare to EMCS)
- Aliphatic spacer offers low potential for eliciting an immune response
